The Veeva Study Training application allows Study Managers, or authorized users, to refine training plans and assignments based on a Learner’s association with a Study, and can further refine the training set by Study Country and Study Site. In addition to standard Training matrix setup, Study Managers will want to set up a few components specific to Study Training.
For example, if the Study Manager wanted participants in a study at the “Dr. Vern & Associates” site to receive site-specific training, they could select the Dr. Vern & Associates Study Site record in the appropriate Curriculum. When Training automation creates Training Assignments, it will check that the Learner is associated with Dr. Vern & Associates before assigning that Curriculum’s set of training requirements.
You can automate many of the setup steps described in this article using the standard Study Training-Clinical Operations Connection.
About Study Training Matching Rules
When a Study Manager adds a Person to a Study, Vault creates a Study Learner Role-Person relationship record which includes a system-managed matching rule. Based on that matching rule, Study Training filters the Curricula associated with the Study Learner Role to only those Curricula appropriate for the Study, Study Country, or Study Site, and assigns them via Training automation.
Setup Overview
Study Managers should perform the following during Study Training matrix setup:
- Create Study, Study Country, and Study Site records to suit your needs.
- Create Study Learner Roles.
- Create Study Curricula and specify Study, Study Country, or Study Site.
- Create any necessary CrossLinks to training documents in other Vaults.
- Create Training Requirements and, optionally, specify Study metadata.
Additional Features
Study Training includes the option to enable the below features.
- The Study Training Matrix Builder allows an authorized user to rapidly assemble the training needs for a given Study, guided by the builder’s intuitive user interface.
- The My Study Team Page provides CRAs and site managers immediate access to real-time insights into the state of a site’s training progress via the My Study Team tab.
- When configured on a document lifecycle, the Create Related Training Requirement entry action automatically creates related Training Requirements.
- Training records captured in Study Training Vaults are key trial artifacts relevant to an organization’s trial master file in Veeva eTMF. To support this relationship and eliminate manual processes for filing training records to eTMF, Vault can automatically file Study Training logs as Library documents, then transfer them to eTMF as CrossLinks.
- Security Profile Mapping further automates user management by allowing new auto-created User records to be associated with a custom security profile instead of the standard Training User profile (
training_user__v). - The Training Assignment object lifecycle can be configured to manually transition assignments to the Resolved state. This supports scenarios such as Learners dropping out of a study with incomplete assignments that should not be marked Cancelled or Completed.
Creating Study-Related Object Records
Study Training uses a combination of metadata in the form of Study, Study Site, and Study Country to match training to the correct Learners. If configured, the Study Training-Clinical Operations Connection transfers study-related data to your Study Training Vault. However, to set up matching for a given study, Study Managers should first create a Study record:
- Navigate to Business Admin > Studies or to a custom Study object tab.
- Click Create.
- Fill in the required details, including a Study Number.
- Click Save.
Once the Study record is saved, the Study Manager should create any necessary Study Country values:
- On the Study record details page, navigate to the Study Countries section.
- Click Create.
- Select a Country record. If the intended value isn’t available, an Admin may need to add it to the Vault.
- Click Save.
- Repeat as necessary for all countries associated with the Study.
Once the Study Countries have been populated, the Study Manager should create any necessary Study Site values:
- On the Study record details page, navigate to the Study Sites section.
- Click Create.
- Enter a Study Site number.
- In the Study Country field, select one of the Study Countries created via the above steps.
- If the site is associated with an organization, select it in the Organization field.
- Click Save.
- Repeat as necessary for all sites associated with the Study.
About Record Transfer & Training Readiness
The Study Training-Clinical Operations Connection transfers study-related data to your Study Training Vault. To ensure ongoing training readiness, the connection’s logic:
- Allows a Study Person record to be transferred without a Study Country or Study Site value.
- Prevents Study Persons from receiving training before their Study Site is ready.
This is useful for handling, for example, multiple CRAs for the same study, where some CRAs must be trained before a Study Site is ready, and others should only be trained after this milestone.
To do this, the nightly connection job reviews Study Country, Study Site, and Study Person records in Clinical Operations. When a record has been modified since the last job run (Last Modified Date) and its Connect to Vault Study Training field is set to “Yes”, the job transfers it to Study Training with the appropriate field values. The connection also references the Ready for Training field in Study Site and Study Person records, then transfers or updates them when the field value is set to “Yes”.
Note: The connection transfers Clinical Operations records only when the job runs. However, it is possible for the job to skip a record in a run, then transfer it in the next. This is an edge case which can occur if a user updates a record and the Clinical Operations Vault has not yet populated the Study Country and Study Site object lookup fields prior to the job run. This operation occurs quickly, but may not complete in time for the job to detect the updates via the record’s Last Modified Date.
Creating Study Learner Roles
Study Training uses the relationship between Study Learner Role and Person to match users with the appropriate training content. Once all Study-related records have been populated, the Study Manager should next ensure that all necessary Learner Roles of the Study Learner Role object type exist in the Vault.
For each Study Learner Role, you must also create a corresponding Clinical Mapping record. This enables the Study Training-Clinical Operations Connection to transfer the relevant records to your Study Training Vault.
A training matrix can include up to 150 Study Learner Roles referencing a single Study.
Creating Study Curricula
In order to work with Study Training matching rules, when Study Managers create Curricula, they must be of the Study Curriculum object type. Each Study Curriculum is associated with a Study, and a Study Manager may also associate it with a Study Country, Study Site, and, optionally, a Study Learner Role.
A training matrix can include up to 200 Study Curricula referencing a single Study.
To create a Study Curriculum:
- Navigate to Business Admin > Curricula or to a custom Study Curriculum object tab.
- Click Create.
- Enter a Name.
- Select a Study. If this is the only study-related value you select in the curriculum, then Study Training will assign this curriculum to all Persons added to that Study.
- Optional: Select a Study Country. Selecting a country causes Study Training to exclude this curriculum for Persons with any other (or no) Study Country value selected when added to the Study.
- Optional: Select a Study Site. Selecting a site causes Study Training to exclude this curriculum for Persons with any other (or no) Study Site value selected when added to the Study.
- Click Save.
- Repeat as necessary to cover all necessary Study, Study Country, and Study Site scenarios.
Note: In Study Training, you cannot have both Study Curriculum and base Curriculum object records associated with the same Learner Role.
Creating Training Requirements for Study Training
Once a Study Manager has created their Study Curricula with appropriate matching data, they can create Training Requirements, populate them with Training Materials, including CrossLink documents. Then, either use them in the matrix builder or add them manually to the appropriate Study Curricula:
- On the Training Requirement object details page, navigate to the Curricula section.
- Click Add.
- Optional: As the number of Study Curricula in a Study Training Vault can be large, use the Filter section to narrow your selection to the Curricula for a particular Study.
- Select the Curricula to which you want to add the Training Requirement.
- Click OK.
Study-Related Data on Study Training Requirements
Study Managers can optionally add Study, Study Country, and Study Site values to a Training Requirement. When Vault creates a Training Assignment from that Training Requirement, it also copies these values over to the Training Assignment. This can be useful for reporting data.
When a document includes a Study value, Training Matrix Automation creates one Training Requirement per referenced Study and populates the study’s name within the record’s Name and Study fields.
It is possible that a new requirement’s related document already has an in-use Training Requirement for a different Study, and that Learners are already assigned or have already completed related Training Assignments for the same document version. This means that a Learner who has already completed an assignment for Study A would be required to complete training for the same document version in Study B once automatically created.
To handle this scenario so that Learners are required to complete only one assignment for a given document version (regardless of its assigned Study), Vault additionally treats these incoming requirements as equivalent to existing ones, and automatically marks any extra assignments as complete. To do this, Vault:
- Creates the new requirement as a substitute Training Requirement, indicated by the Cross-Study Substitute field.
- Adds a substitute rule to associate the new substitute with the existing primary Training Requirement.
- Creates and assigns a Training Assignment.
- When the existing assignment is complete, Vault immediately marks the new, substitute assignment as Completed and sets the Completion Source to “Cross-Study Autocompletion”.
- When a Learner’s existing assignment is not yet complete, they can still see this assignment as expected and are required to complete it only once. Once complete, Vault marks the substitute assignment as Completed with Completion Source as “Cross-Study Autocompletion”.
Adding CrossLinks as Training Materials
Study Training allows you to add CrossLink documents from other Vaults, such as an eTMF Vault, to Training Requirements as Training Materials. Ensure that the CrossLink is in a training-eligible lifecycle state, and that the source binding rule is set to Specific Document Version. If configured, the Study Training-Clinical Operations Connection will create CrossLinks for appropriate documents in your Study Training Vault automatically when such documents reach their steady state.
Updating CrossLink Training Materials
To update a CrossLink training document, follow these steps:
- In the Study Training Vault, perform the Create Draft action on the CrossLink document.
- In the source Vault, update the source document.
- In the Study Training Vault, move the CrossLink document to the Approved lifecycle state.
Updated CrossLink documents can trigger Training Requirement Impact Assessments just like documents that reside in the Study Training Vault.
Assigning Persons to a Study
As the final step of Study Training matrix setup, Study Managers should assign Persons to Studies, allowing automation to determine which Study Curricula to assign. Study Training supports small Studies with up to 100 Persons and large Studies with 100 to 1,000 Persons when the Increase Classroom Training Maximum Roster Count application setting is enabled. To allow Study Managers or other authorized users to assign Persons to a Study, a Vault Admin should add the LearnerRole-Person object to the Study object page layout.
To assign Persons to a Study:
- Navigate to Business Admin > Studies or to a custom Study object tab and click into a Study record.
- In the Study Persons section, click Create.
- Select a Learner Role.
- Optional: Select a Study Country.
- Optional. Select a Study Site.
- Click Save.
- Repeat as necessary to assign additional Persons to the Study.
Once you add the Learner as a Study Person, the next time the training automation job runs, it will assign a set of training filtered down to their Study, Study Country, or Study Site.
Creating Role Dependency Records
Study Training can automatically create User Role and User Role Setup records based on the user’s Study Learner Role. This ensures that users, such as CRAs, can see the right information based on their study team role, study, country, or site.
To use this feature, you need to create Role Dependency records for Learner Roles and Application Roles:
- Navigate to Business Admin > Study Learner Roles or to a custom Study Learner Roles object tab and click into a Study Learner Role record.
- Under Role Dependencies, click Create.
- Select a Study Level for the secondary role: Study.
- Select a Target Application Role.
- Click Save.
Role Dependency Use Case
The following steps describe an example use case alongside the preconditions and process by which Study Training auto-creates records at the Study level:
- A Training Admin creates Role Dependencies for a Learner Role and populates the fields as follows:
- Target Application Role: Trainee
- Parent Training Role: CRA (This refers to a CRA Learner Role record.)
- Study Level: Study
- The selected Trainee Application Role record has an assigned Permission Set called Training: Learner User Actions.
- The Training Admin assigns a user (Person) to the CRA Learner Role for the CHLCAP123 Study.
- Study Training automatically creates User Role Setup records based on existing Role Dependency data via hourly jobs. In our example, each job performs the following:
- The Clinical to Study Training User Management job creates a User Role record for the user for the Trainee Application Role.
- The Study Training: Sync Security Records job creates a User Role Setup record for the user for the CRA role in the CHLCAP123 Study.
- The user’s permissions are automatically limited to those defined in the Trainee Application Role’s permission set, Training: Learner User Actions.
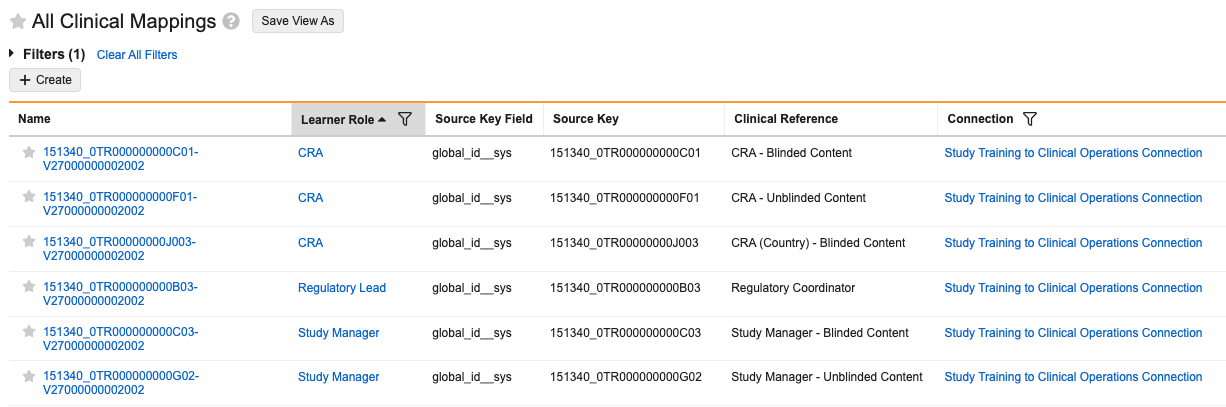
Clinical Mappings
Clinical Mapping (study_team_role__v) records are used in your Study Training Vault to map Learner Roles (study_learner_role__v) to the various project team and site staff roles and responsibilities defined in your Clinical Operations Vault. These mappings allow Study Training Admins to quickly set up a study’s training matrix in the Study Training Matrix Builder.
To align roles and responsibilities across Vaults, Study Training automation references various Clinical Mapping record fields:
- Source Key Field locates the relevant Clinical Operations record. Roles are identified via
global_id__sys, and responsibilities are identified viasystem_id__v. - Training Type indicates the mapping is for a Role or Responsibility.
- Role Type indicates whether the role or responsibility is for Project Team or Site Staff members.
Note: Prior to 23R2, the Clinical Mapping (study_team_role__v) object may have been labeled in your Study Training Vault as “Study Team Role” or “Clinical Study Team Role”. See Configuring the Study Training-Clinical Operations Connection for more information about this object’s history and purpose, as well as the required configuration to begin using Clinical Mappings for roles and responsibilities.
Learner Roles
Learner Roles (study_learner_role__v) in Study Training are mapped to Study Team Roles assigned to Study Persons in your Clinical Operations Vault. Multiple roles in Clinical Operations can be mapped to a role in Study Training. For example, CRA - Blinded Content and CRA - Unblinded Content in Clinical Operations can both map to a CRA role in Study Training. However, a Study Team Role in Clinical Operations cannot be mapped to multiple learner roles in Study Training.
To map a Learner Role in Study Training:
- Navigate to the Clinical Mapping (
study_team_role__v) object in Business Admin or in a custom tab. - Click + Create.
- Select a Learner Role from the dropdown. The records you can select are limited to Learner Role (
study_learner_role__v) records, a Learner Role object type. - Populate the Training Type (Role) and Role Type (Project Team or Site Staff) fields.
- In the Connection field, select Study Training to Clinical Operations Connection.
- In the Source Key Field Selector field, select
global_id__sys. Vault populates the selection as the Source Key Field.- Vault identifies the corresponding Clinical Operations record via
global_id__sysonly. - You cannot configure a custom field or select a different standard field to use as the Source Key Field.
- Vault identifies the corresponding Clinical Operations record via
- In the Source Key field, enter the source key field value for the corresponding Clinical Operations record.
- Click Save.
Responsibilities
Study Training uses Clinical Mapping records to map the various Study Person Responsibilities a Study Person is granted via the Delegation of Authority (DOA) log, for example the abilities to administer informed consent, perform a physical exam, or administer study drugs.
You cannot manually create, edit, or delete Clinical Mappings for responsibilities in your Study Training Vault. Instead, Veeva provisions records to both Vaults as part of a Vault release. Upon upgrade, the following records are available:
- In Clinical Operations, standard Responsibility records track the task itself, for example Administer Scales. These records are available for selection as Study Person Responsibilities when adding a Person to a Study (creating a Study Person record).
- In Study Training, each Clinical Operations Responsibility corresponds to a Clinical Mapping record with its Training Type field set to “Responsibility”.
When you create any additional custom responsibilities in Clinical Operations, the Study Training Vault automatically creates a corresponding Clinical Mapping record via the connection’s inbound Integration Points once the Study Person Responsibility is assigned to a Study Person.
Security Profile Mapping
By default, when the Study Training-Clinical Operations Connection auto-creates domain users, the Study Training Vault assigns these users to the standard Training User Security Profile (training_user__v), and a Vault Admin must manually assign a different security profile to certain users to perform certain tasks. For example, when a new user is also a Study Manager, the auto-created User record’s Security Profile field must be manually updated to a custom “Training: Study Manager” profile.
When the appropriate Security Profile Mapping records are in place, the Study Training Vault can instead automatically add new users to an Admin-defined Security Profile. This eliminates any manual Vault Admin steps for a majority of new Study Training users.
Enabling Security Profile Mapping
To use this feature, a Vault Admin must minimally:
- Within the Security Profile Mapping object, enable the Display in Business Admin option, or otherwise create a tab referencing the Security Profile Mapping object.
- Ensure any users working with Security Profile Mapping records are assigned a permission set with at least Read, Create, and Edit object permissions. Delete permission is optional.
Creating Security Profile Mappings
To create Security Profile Mapping records:
- In the Clinical Operations Vault, navigate to the relevant Security Profile in Admin > Users & Groups > Security Profiles > [Security Profile].
- Ensure the profile’s Vault URL is fully visible in your browser window, for example
https://vern-bio.veevavault.com/ui/#admin/users_group/permission_sets/detail=&d=ctms_site_monitoring__c - Copy the profile’s key at the end of the URL, for example
ctms_site_monitoring__cin the URL above.
- Ensure the profile’s Vault URL is fully visible in your browser window, for example
- In the Study Training Vault, navigate to Business Admin > Objects, then locate and select the Security Profile Mapping object.
- Click + Create.
- In the Clinical Security Profile field, populate the profile key you copied in Step 1.
- In the Training Security Profile field, select the desired security profile.
Repeat this process for each profile as required.
Note: This feature only supports creating Security Profile Mappings where multiple Clinical Operations profiles map to a single Study Training profile. You cannot map a single Clinical Security profile to multiple Study Training profiles. See Limitations below for additional considerations.
Limitations
Security Profile Mapping is subject to the following limitations:
- This feature specifically supports creating new Study Training domain users only.
- Vault does not update existing User records to accommodate new Security Profile Mapping records.
- VeevaID users are not supported. These users are handled via a different job, VeevaID Invitation Management.
- Security Profile Mapping only applies to Clinical Operations users associated with a connected Study, meaning a given user must have a Clinical Person record associated with a Study record where the Connect to Veeva Study Training is “Yes”. This additionally means that admin-type users which exist only in Study Training must still be manually created.
- An important caveat to Limitation 1 above: This feature introduces syncing domain user statuses across Vaults. Along with Study Training’s Study Training ClinOps: User Integration Rule and related field rules, the Clinical to Study Training User Management job processes updates to Clinical user statuses, meaning that when a Clinical User record’s Status changes to active or inactive, the job updates the Study Training User accordingly.
- This feature only supports creating Security Profile Mappings where multiple Clinical Operations profiles map to a single Study Training profile. You cannot map a single Clinical Security profile to multiple Study Training profiles.
Training Assignment Lifecycle Resolved State
The Training Assignment object lifecycle includes an optional Resolved state, allowing Training Admins to manually transition assignments to this state when other terminal lifecycle states such as Cancelled or Completed do not apply. This provides a more accurate representation of an assignment’s status in situations when, for example, a Learner drops out of a study with incomplete assignments, or a Learner was mistakenly assigned training that should be acknowledged as invalid without cancelling it. The Resolved state can also be useful for appropriately marking assignments after Vault processes a TRIA decision.
The Resolved state can optionally be used with the Training Assignment object’s Resolution Reason picklist field: When a Training Admin marks an assignment as Resolved, Vault prompts them to capture a reason such as Assigned in Error, Left Study Before Completed, or On Leave.
Configuring the Resolved State
To configure this capability:
- Navigate to Admin > Configuration > Objects > Training Assignment > Actions and create a new object action using the VT: Change State to Resolved action. During configuration, do not select the “Available in All Lifecycle States” option. These instructions include steps for configuring this as a user action on the Training Assignment lifecycle’s Assigned state.
- Navigate to Admin > Configuration > Object Lifecycles > Training Assignment Lifecycle > Assigned.
- To allow users to execute this action for a single Training Assignment record: Configure a user action using the VT: Change State to Resolved action you created in Step 1. While we recommend configuring the “Change State to Resolved” action to always appear in the lifecycle’s Assigned state, the action’s label, configured states, and conditions can be updated to accommodate your organization’s requirements. For example, you can configure the action on this state conditionally, such that it is only available when the record’s Creation Source field is System, Direct Assignment, or Self Enrollment. This aligns with a limitation of this feature, which only allows a state change to occur under these conditions.
- To allow users to execute the action in bulk, create a second user action using the Change State to action. This should be configured within the same lifecycle state(s) selected for the single-record action, and we recommend using a similar label such as “Change State to Resolved (Bulk)”.
- Optional: Navigate to Admin > Configuration > Objects > Training Assignment > Fields and configure the Resolution Reason field’s picklist values according to your organization’s requirements.
- When this picklist is configured with active values, it automatically appears for Training Admins performing the single-record state change described above, without any additional configuration. Resolution Reason cannot be captured when changing state in bulk. See Limitations for details.
- If your Study Training Vault was created on or after the 25R3 release, the following picklist values are included, but can be re-labeled or inactivated as required: Assigned in Error, Left Study Before Completed, and On Leave.
- You can optionally configure the Resolution Reason field on all applicable Training Assignment object page layouts. We recommend including it near the Completion Date field.
- Review the Training Assignment object lifecycle’s atomic security and role configuration to ensure Training Admins and other roles are granted the appropriate permissions.
Limitations
The Resolved state is subject to the following limitations:
- The Training Assignment object’s Resolution Reason field configuration must remain such that it is optional. When the field is configured with the User must always enter a value (required) option, all Training Assignment creation will fail. This is because Vault attempts to set required fields when creating records, and the Resolution Reason is captured after assignment creation. Further, the field’s requiredness configuration does not extend to the Resolution Reason value Training Admins select when marking records.
- The Resolution Reason cannot be captured when performing the action in bulk. Instead, Training Admins can first perform the bulk state-change action to Resolved, then perform a second bulk action to update the Resolution Reason field.
- Assignments in the Resolved state do not appear on the Learner Homepage.
- An assignment can only be moved to the Resolved state when:
- Its Creation Source is System, Direct Assignment, or Self Enrollment.
- It is in an open state, such as Assigned. Vault ignores assignments in terminal states, such as Completed or Cancelled.
Enabling the My Study Team Page
The My Study Team page provides CRAs and site managers immediate access to real-time insights into the state of a site’s training progress.
To enable the My Study Team page:
- Review your Vault’s tab configuration in Admin > Configuration > Tabs and Tab Collections.
- The My Study Team tab is delivered to your Vault as Active, however you can optionally update the Label that users will see. If you relabel this tab, we also recommend updating the page label in Admin > Configuration > Pages.
- If your Vault is configured to use Tab Collections for Study Training, add the My Study Team tab to the desired collection(s).
- Review your Vault’s security configuration for Study Training functionality. To use the My Study Team page, users must be assigned a permission set with the below permissions.
Limitations
The My Study Team page only displays assignment counts for:
- Assignments Vault creates as part of the Learner’s training matrix, according to the assignment’s Creation Source field. The My Study Team page does not count Training Assignments created as a result of Direct Assignment or Self-Enrollment.
- Learners who are Vault users, according to their Person record.
- The My Study Team page does not count assignments for Learners with the Person is not a Vault user field selected. This is because Vault only counts Training Assignment records with a Manager Group assigned to the Direct Manager role. Vault does not create or populate Manager Groups for Learners without a User record.
- Assignment counts exclude Learners who are not Vault users. Additionally, these Learners cannot receive nudges.
Related Permissions
Study Managers require a security profile with the following permissions to create and manage a Study Training matrix:
- Read, Create, Edit, and Delete permissions for the following Study Training objects:
- Study
- Study Country
- Study Site
- Read, Create, Edit, and Delete permissions for the standard Training matrix objects.
My Study Team Page Permissions
To use the My Study Team page, users must be assigned a permission set with the below permissions.
Additionally, if your Vault’s Training Requirement object uses Custom or Matching Sharing Rules, users must be able to access any records they are attempting to select to mark training complete.
| Permission Label | Permission |
|---|---|
| Application: Vault Actions: Workflow | Start eSignature Participate or Query permission |
| Objects: Assignment Details | Read |
| Objects: Media: Object Permissions: Image | Read |
| Objects: Nudge Detail | Read |
| Objects: Nudge Details User Input | Read |
| Objects: Person: Object Field Permissions | Read for the following object fields: ID Image Manager Name Training Eligibility Status User ( vault_user__sys) |
| Objects: Facilitated Training Request: Object Permissions | Read, Create, Edit for all object types. Note: Users must also have Read permissions for any required Facilitated Training Request object fields. These may be configured in this object’s lifecycle, or in the permission set (Object Field Permissions). |
| Tabs: My Team | View Note: If this tab is configured in a Tab Collection, Managers also require View access to that collection. |
| Pages: My Team Page | View |
Resolved State Permissions
To use the Training Assignment object’s Resolved state, the Training Assignment object lifecycle must be configured such that:
- The Training Administrator and Owner roles are permitted Edit access in the Resolved state. All other roles with Read access in other states should be granted Read permission in this state.
- Within the configured state (Assigned):
- The Resolution Reason field is defaulted to Read, and the Training Administrator role has Edit permission via role override.
- Both the individual and bulk Change State to Resolved actions are defaulted to Hide, and the Training Administrator role has Execute permission via role override.